Login (User Login; top right of page) with your web user or complete this form to download.
Romaco: Integration and flexibility for packaging of pharmaceuticals (Italy)
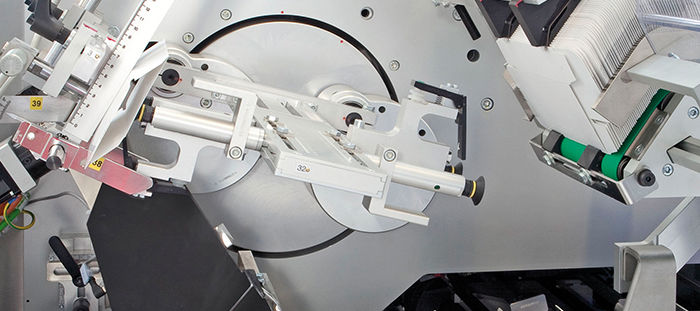
Romaco Packaging, a division of the Romaco Group, develops and manufactures machines for filling and packaging. In line with the Group's philosophy, expressed in the motto, “Excellence through innovation,” Romaco Packaging has always invested in offering its customers machines that are ergonomic and simple to use. “We have always paid a great deal of attention to the human-machine interface,” recounted Lino Bagnacavalli, Electronics Manager at the Romaco Macofar Division. “As early as 1999 at the Interpack Trade Fair we presented a unique interface with a singular display for all of the machines made by the group, manufactured by companies that were then much less integrated than what is the standard today. However, in 2005, when Macofar had to develop a solution for the first time corresponding to the new requirements coming from the pharmaceutical industry supervisory agencies, our technicians were forced to use a new dedicated system that was much more sophisticated, but also much more costly. So, from that time onwards, we began to think about an alternative solution for the future. Immediately, zenon seemed competitive, because of its reduced costs, its distribution, its flexibility, its high level of openness and its simplicity. We believed that such a solution would have even enabled us to integrate other applications into the HMI and to use one, single software in all of the group's machines.”
After due reflection, in 2009, Romaco engineers decided to re-open the discussion on human-machine interfaces, once again following a request by a customer working in the pharmaceuticals industry, who needed a machine complying with the FDA Standard 21 CFR part 11.“It was then that we decided to field test zenon's potentials. The trial was a success. Afterwards, even the Promatic division developed an application with zenon for a customer that needed the same FDA approval. In 2010 we built the first line that integrated a production supervision SCADA. In this highly complex implementation, the machines were connected in a network using a server to download the formulas, manage batches and create a data backup. Again, zenon proved to be an excellent solution because of its flexibility and its ease of use both for the simpler HMI applications as well as for the most complex applications with supervision functions.”
Compliance and connectivity
In the pharmaceuticals industry, the HMI system plays a crucial role in guaranteeing compliance. Time synchronization of data and a series of other functions offered by zenon enabled Romaco to extend the capabilities of its machines to offer full compliance with the regulations set out by regulatory authorities.
For the management of production lines with several machines, for example, Romaco was able to introduce unified user access management. “This is an extremely useful function that simplifies operator tasking,” Bagnacavalli pointed out. “It may seem trivial, yet, often even on a single line with machines from the same manufacturer, operators have to login with a password at the different stations.”
By choosing zenon even backup management and report generation were made easy. Help came even for the supervision application. “With zenon the production manager can view a display of all the machines simultaneously on one monitor.”
Another important aspect for Romaco was zenon's openness to communicate with outside management systems and its ease of integration with different control systems.
For interface functions zenon proved to be superior to the previous solutions while also fitting in perfectly with Romaco's current requirements. “Choosing zenon was an investment for our future: with this system there are no limits to future expansion of functions to be added.”An example: “We believe that the ability to interface the shop-floor with management systems such as SAP ERP, is one of the features we will implement. In the near future this will provide an important competitive advantage.”
Integrated identification
In particular, Romaco appreciates zenon's openness and its ability to dialogue and integrate with other systems. “This software's open and flexible structure enabled us to integrate third party hardware and software into our machines, giving us new functions, such as reading identification codes and printing, which had previously only been covered by separate stand-alone systems. At the same time, everything was in compliance with current regulations in Europe and the United States, relates Franco Ficarra, Electronics Manager at Promatic, the Romaco Group division that makes cartoning machines and case packers: “Promatic was the first company in the group to take advantage of zenon's capabilities. From the very beginning we were convinced that we could take full advantage of this software's potentials to bring machine management together into a single system, with one single HMI display panel and the code reading system, which up to then had worked independently with its own monitor,” Ficarra pointed out. The first integrated application was made for a Swiss customer. “We developed a simple, ergonomic machine, with a single PC display capable of controlling all functions including the Laetus laser scanner, ensuring compliance with 21 CFR part 11: a single formula for both, machine and laser scanner parameters, one audit trail and a single user log-in.” That's without mentioning the performance: “By integrating all of the functions we have been able to not only obtain an economic benefit but also an advantage in terms of the speed of our machine as it can read 400 datamatrix codes per minute.”
This extremely positive experience laid the foundation for further integrations. “Because of zenon our technicians are now able to add new functions to our blister pack machines, such as for example, a quality control viewing system or a laser printer. Whilst our competitors are offering “closed” systems into which new functions are added by adding external modules downstream of the process, we have been able to integrate everything into one single environment making us capable of responding to our customers' requests with flexibility.”
Also in Germany Romaco chose to use zenon for its HMI systems on the blister pack and sachet filling machines: “In this industry there is a pressing need for integration in order to offer users a unified management system for all the machines on a line, for audit trails and for formulas.” Therefore, there will be cooperation between Karlsruhe, Germany and Bologna, Italy to share experiences and to standardize their displays. In short, a future that is zenon-proof.
In Germany and Italy
Romaco Packaging brings together the business of six brands: Bosspack, Macofar, Noack, Promatic, Unipac and Siebler. This business unit has offices in Karlsruhe, in Germany, where the Group's headquarters are as well, and offices in Rastignano, in the Bologna Province. In Germany, Romaco manufactures solutions under the Noack, Bosspack and Siebler brands for tablet and capsule counting and bottle filling, as well as blister pack and sachet filling machines. In Bologna, on the other hand, Macofar machines for filling bottles with liquids and for sterile powder dosing are developed; then there are Unipac machines for tube filling and Promatic machines for cartoning and case packers. Romaco Packaging focuses on the pharmaceutical industry, which must comply with very strict supervision by American and European regulatory authorities.
An industry with strict rules
The machines manufactured by Romaco Packaging are for the most part intended for pharmaceutical companies with manufacturing facilities located all over the world, specifically, in South America, India and Eastern Europe, where a large part of the pharmaceutical manufacturing intended for European and American markets has been delocalized.
The pharmaceutical industry, as is known, undergoes rigorous regulation both in Europe and in America. Reference regulations for the United States are contained in the well-known standard 21 CFR (Title 21 of the Federal Food & Drug Administration Code). FDA regulations were designed to protect consumers. They require drug manufacturers to organize their manufacturing processes in such a manner as to always be able to clearly identify the ingredients, formulas and machinery that were used for the production of each batch of pharmaceutical drugs. This way they are able to identify any factors that may have contributed to an error.
Back